- Daniel Kremens, MD, JD, FAAN
- Associate Professor of Neurology
- Sidney Kimmel Medical College at
- Thomas Jefferson University
- Co-Director of the Parkinson's Disease
- and Movement Disorders Division
- Sidney Kimmel Medical College at
- Thomas Jefferson University
- Philadelphia, Pennsylvania
Educational Objectives
- Discuss the significance of motor fluctuations in patients during "wearing OFF" periods with current PD therapies
- Implement strategies to prevent and manage motor fluctuations in patients with PD
- Describe new and emerging pharmacologic strategies to treat "OFF" episodes
Section 1: Strategies for optimal management of motor complications in Parkinson's disease

Case presentation: Brian is a 63-year-old man diagnosed 5 years ago with Parkinson's disease (PD). At diagnosis, he began taking carbidopa-levodopa 25-100 mg 3 times per day. Despite initial symptom benefit with levodopa for the first few years, Brian reports that he has been experiencing significant "OFF" episodes over the past year that now occur 2 to 3 times a day. He reports symptoms of tremor, problems with balance, and clumsiness, as well as noticeable changes in mood and difficulty sleeping. He feels that he is not as "sharp" mentally as he used to be. About a year ago, he increased his dosage to 4 times per day on his own, with some modest improvement, but he continues to have delayed ON as well as dose failures. In particular, he notes that his mornings are especially bad as he waits for his first dose of carbidopa-levodopa to take effect. The OFF episodes and psychological issues are having a significant deleterious effect on his activities of daily living.
Introduction
Most patients with long-term PD ultimately experience signs and symptoms of levodopa-induced motor fluctuations, nonmotor symptoms, and/or dyskinesias. These symptoms can be fluctuating, unpredictable, and disabling. Motor fluctuations may include tremor, weakness, balance problems, and other symptoms that interfere with daily activities, while nonmotor symptoms may range from bodily signs (eg, gastrointestinal changes, urinary incontinence, excessive sweating, orthostatic hypotension) to psychological or cognitive changes (eg, anxiety, depression, panic, speech difficulty, and others).
With disease progression, cumulative OFF time may ultimately occupy up to 50% of a patient's waking hours. A major contributor to total daily OFF time is morning akinesia as patients wait for their morning levodopa dose to be absorbed, cross the blood-brain barrier, and take effect. Morning akinesia typically results from either delayed ON or dose failures. These are believed to be the result of delayed gastric emptying (gastroparesis), intestinal protein competing with medication absorption, and other pharmacodynamic factors. Increasing the levodopa dose or adding adjunctive therapies may reduce end-of-dose OFF episodes, but these strategies do not control delayed ON, dose failures, or suboptimal ON response because no oral therapy can accelerate the onset of levodopa.1 It is apparent that therapies with different mechanisms of action bypassing the gastrointestinal tract are needed to treat patients with PD and troublesome motor fluctuations.
You may see many patients like Brian in your practice because his problems with levodopa treatment are all too common. Part 1 of this activity will cover the signs and symptoms, impact on quality of life, and management of motor fluctuations. Part 2 will discuss the pathophysiology of motor fluctuations, recently approved agents for OFF episodes including on-demand rescue therapies, emerging treatments, and strategies for community neurologist health care providers (HCPs) who care for patients with PD.
Expert Video Commentary
Significance of Motor Fluctuations in Patients with PD
Definitions
PD is a chronic, progressive neurodegenerative disorder that affects dopamine-producing neurons in the brain, specifically in the substantia nigra, resulting in resting tremor, bradykinesia, limb rigidity, and problems with gait and balance.2 About 10 million people worldwide and 1 million people in the United States have PD.3
Levodopa is the gold standard for treatment of PD because it is highly effective. However, most patients taking levodopa will experience motor fluctuations and/or nonmotor symptoms due to a return of symptoms as each dose wears off, a delay in medication onset of action, and fluctuations in medication concentrations (Figure 1).4,5 Resulting signs and symptoms include wearing OFF episodes (motor and nonmotor symptoms) as well as dyskinesias (involuntary movements), dystonias (painful muscle contractions), or freezing episodes.3,5,6
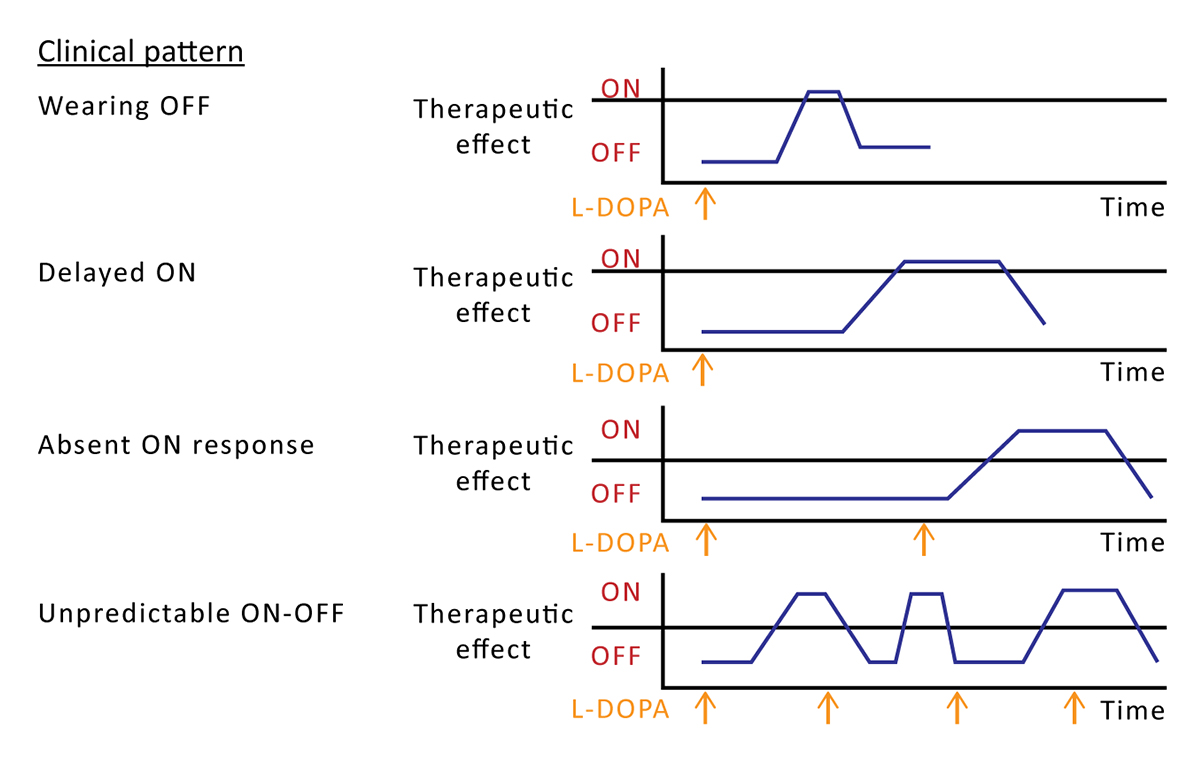
Figure 1. Effects of varying plasma levodopa concentrations on motor fluctuations in levodopa-treated Parkinson's disease.4
Prevalence
Signs and symptoms of wearing OFF vary among patients.5 The prevalence of motor fluctuations, nonmotor symptoms, and dyskinesias also depends on disease duration, and severity increases along with the duration of PD.7,8
Wearing OFF periods have been reported in about 57% to 67% of patients with a mean PD duration of 6 years, as identified by neurologists or patients themselves; the rate reached 80% by self-report in patients with >10 years' disease duration.7,8 Other data indicate rates of motor fluctuations in 60% to 90% of patients after 5 to 10 years of PD treatment.9 Even patients with early disease may experience OFF symptoms. A questionnaire survey revealed wearing OFF symptoms in 42% of PD patients with less than 2.5 years' disease duration.7
Other types of motor fluctuations have similar prevalence rates. Nonmotor symptoms occur in 60% to 97% of PD patients.6 Dyskinesias occur in about 50% to 80% of all patients taking levodopa for 10 years or more.8,9 However, dyskinesias may also occur within weeks of beginning levodopa therapy.10
Role of COVID-19
Among the recent challenges of PD management is SARS-CoV-2, the novel coronavirus. Patients with PD may have specific risk factors for contracting COVID-19 (such as older age) and a more difficult time recovering from it because of changes to the immune system.11 Data indicate that patients with PD and COVID-19 infection have a significantly increased case fatality rate compared with patients with COVID-19 without PD; risk was increased independent of age, sex, and race.12 Further, a community-based observational study showed that patients with PD and COVID-19 had significantly increased motor and nonmotor symptoms and daily OFF time compared with uninfected patients, independent of age and PD duration. Although it is unclear whether the difference was due to a systemic inflammatory response or direct invasion of the central nervous system, the changes were sufficient to warrant increases in dopaminergic therapy.13
Characteristics of Different OFF Episodes and Failure of ON Response
There are several types of motor fluctuations and dyskinesias that may occur with long-term levodopa treatment; specific types are described in relation to the medication dose (Table).3,14,15
| Motor fluctuations |
|---|
| End-of-dose or "wearing OFF" episodes |
| Unpredictable or sudden ON/OFF episodes |
| Early-morning OFF with hypokinesia or akinesia |
| Short-duration ON or OFF freezing |
| Delayed ON |
| Absent ON response (dose failure) |
| Dyskinesias |
| Peak-dose dyskinesia |
| Diphasic dyskinesia |
| OFF period dystonia |
| Table. Motor Complications of Levodopa Therapy in Parkinson's Disease3,14,15 |
Patients may have several hours of OFF symptoms per day,16 increasing the amount of time they may experience troublesome symptoms. Freezing of gait, or the sudden inability to walk, is a major risk factor for falling. It is usually correlated with disease severity but can occur early in the disease course.17 Other nonmotor symptoms also increase the risk of falling, including lightheadedness due to orthostatic hypotension, cognitive dysfunction, vision problems, muscle pain, depression, and anxiety.17 Delayed gastric emptying is a nonmotor symptom of PD that can contribute to delayed ON.16 Dyskinesia typically occurs with a high levodopa dosage (especially at peak dose), although it may sometimes occur at both the peak plasma levodopa concentration and when a dose is waning (ie, diphasic dyskinesia).3,14
Clinical Manifestations of Motor Fluctuations
Motor symptoms include tremor, weakness, balance problems, slowness, reduced dexterity, stiffness, muscle cramps, and difficulty speaking.7 OFF periods due to motor symptoms affect dressing, hygiene, mobility, housekeeping, eating, writing, and other basic activities.18
Nonmotor symptoms encompass a wide range of manifestations including neuropsychiatric, autonomic, and sensory features such abdominal discomfort, anxiety, apathy, bowel dysfunction, cognitive changes, depression, excessive sweating, hallucinations, impulse control problems, mood changes, numbness, orthostatic hypotension, pain, panic, sleep disorder, speech difficulty, sexual dysfunction, urinary incontinence, and vision changes.3,6,7
Both motor and nonmotor symptoms may manifest as wearing OFF,7 and both motor and nonmotor symptoms can have a fluctuating pattern.6 Nonmotor symptoms may precede the re-emergence of motor symptoms during wearing OFF episodes.5
Impact on Function, Quality of Life, and Caregivers
Wearing OFF symptoms (motor and nonmotor) have a significant negative effect on quality of life.7 A survey of 60 patients with OFF episodes and 60 caregivers showed that increasing severity of PD and a higher number of OFF episodes per day both significantly impaired quality of life; patients expressed the need for better therapies to reduce OFF symptoms and improve quality of life.15
A literature review identified the need for a better understanding of the lived experiences of patients with OFF symptoms and their caregivers.18 Caregivers have an added burden of caring for patients with PD during OFF episodes, especially as the disease progresses, for example providing physical assistance, ensuring their loved one's safety during freezing episodes, facilitating communication with HCPs, and dealing with their own psychosocial challenges.3,18,19
Nonmotor symptoms also have a negative effect on quality of life and are generally underappreciated.6 Neuropsychiatric symptoms in particular impair quality of life for patients and increase caregiver burden, especially if symptoms progress to dementia, impulse control disorders (eg, compulsive gambling, hypersexuality), paranoid delusions, or psychosis. Management options are generally the same as for patients without PD, yet some of the common therapies used for neuropsychiatric symptoms worsen PD motor symptoms.20
Evaluation and Assessments
Several questionnaires have been used in clinical studies to evaluate motor symptoms and the effects of OFF episodes on quality of life, including both generic and PD-specific instruments. HCPs may find it helpful to familiarize themselves with the motor scale of the Unified Parkinson's Disease Rating Scale (UPDRS) and the revised Movement Disorder Society UPDRS (MDS-UPDRS) because they are common efficacy endpoints in clinical trials of therapies for motor fluctuations.21–23 To detect all clinically relevant episodes in clinical practice, HCPs should ask patients about OFF episodes in terms of frequency and severity and engage patients in shared decision making about treatment strategies.18
Initial Management Strategies for Motor Fluctuations
Treatment of OFF episodes requires consideration of the patient's degree of disability, efficacy of the PD therapy, potential adverse effects, and the need to prevent long-term motor complications.24
Adjusting the Dose or Formulation of Levodopa
The initial strategy for OFF episodes is adjusting the dose of levodopa: using a higher dose, giving the same dose more frequently, or using a smaller dose more frequently.3 It may help to take levodopa on an empty stomach to enhance absorption.24 It should be noted that avoiding levodopa as initial treatment (ie, using monoamine oxidase B [MAO-B] inhibitors or dopamine agonists instead) in an effort to prevent OFF episodes has little effect on long-term outcomes.25
Carbidopa-levodopa controlled release
Carbidopa-levodopa controlled release (CR) was developed to provide more sustained levodopa plasma concentrations when compared with immediate-release (IR) carbidopa-levodopa. However, this formulation results in unpredictable (and often incomplete) absorption and delayed clinical benefit; CR carbidopa-levodopa offers only a small improvement in clinical benefit compared with IR carbidopa-levodopa, and it does not decrease the incidence of motor fluctuations.26
Carbidopa-levodopa extended release
Carbidopa-levodopa extended release (ER) is a combination of IR and ER beads intended to provide sustained therapeutic effect and avoid pulsatile release of dopamine; it is approved by the Food and Drug Administration (FDA) for reducing OFF time in PD.27 Carbidopa-levodopa ER has been shown to improve OFF time compared with carbidopa-levodopa IR and has been deemed clinically effective and safe.24
Carbidopa-levodopa intestinal gel infusion
Carbidopa-levodopa intestinal gel infusion is an enteral (liquid) suspension of carbidopa-levodopa indicated for the treatment of motor fluctuations in advanced PD.28 A jejunal pump provides continuous carbidopa-levodopa delivery, which results in more stable dopamine plasma concentrations compared with oral levodopa.6,24,28–30 Gel infusion can reduce OFF time, improve ON time, and reduce dyskinesias.24,30
Using Adjunctive Medications
Dopamine agonists
Adjunctive dopamine agonists (eg, pramipexole, ropinirole, rotigotine) have a longer half-life than levodopa and act directly on dopamine receptors in the brain.14,24 Adding a dopamine agonist may decrease pulsatile stimulation of dopamine receptors and thereby reduce motor complications compared with levodopa.24,31
MAO-B inhibitors
Enzyme inhibition with adjunctive use of MAO-B inhibitors (eg, selegiline, rasagiline, safinamide) is effective for reducing motor complications.14,24,30 MAO-B inhibitors work to antagonize the breakdown of dopamine by monoamine oxidase. They improve the dopamine-deficient state and have anti-glutamatergic effects, and they reduce OFF time when added to levodopa.32 A systematic review documented the efficacy of adjunctive rasagiline, safinamide, and selegiline vs placebo, with the greatest efficacy found for selegiline combined with levodopa.33
COMT inhibitors
Enzyme inhibition with adjunctive use of catechol-O-methyl transferase (COMT) inhibitors (eg, entacapone, tolcapone, opicapone) also reduces motor complications.14,24,30 COMT inhibitors extend the half-life of levodopa by inhibiting an enzyme that metabolizes levodopa. This action increases the bioavailability of dopamine and may reduce the risk of pulsatile receptor stimulation, allowing dose reduction of levodopa.14,31 Entacapone inhibits COMT in the periphery and tolcapone inhibits COMT both peripherally and centrally. Tolcapone is more effective but has been associated with potentially fatal hepatotoxicity, so its use is limited.14 Opicapone is a newly approved COMT inhibitor that is discussed below in Section 2, Adjunctive Therapies.24
Management Strategies for Dyskinesias
Levodopa dose reduction is often sufficient as an initial measure to reduce the occurrence of dyskinesias, but as PD advances, dyskinesias can occur despite peak plasma levels of levodopa (ie, peak-dose dyskinesia).14 Specific therapy for peak-dose dyskinesia begins with reducing each levodopa dose and adding dopamine agonists or amantadine.10
Adjunctive amantadine (Gocovri) is FDA approved in an ER formulation for the treatment of levodopa-induced dyskinesia in PD.3,30 Gocovri provides high plasma concentrations of medication throughout the day and has been shown to reduce both dyskinesias and OFF time versus placebo over 12 weeks.34 This medication is taken at bedtime and controls dyskinesias upon waking and throughout the day.29
Deep brain stimulation may be considered in refractory cases of levodopa-induced dyskinesias.10
Additional Strategies for Management of Motor Complications
Many patients with OFF episodes require rescue therapies that bypass the gut and do not involve oral administration of levodopa.16
Subcutaneous Apomorphine Injection
Apomorphine injection is an on-demand medication indicated for the acute intermittent treatment of hypomobility and OFF episodes (end-of-dose wearing OFF and unpredictable ON/OFF) in advanced PD. This medication is delivered by injection pen.35 Intermittent subcutaneous injections of apomorphine are effective for OFF periods that require rapid reversal.24 Subcutaneous apomorphine injection reliably restores the ON state, similar to that achieved with levodopa.36 Onset of effect with subcutaneous injection is approximately 3 to 12 minutes.3
Apomorphine subcutaneous injection significantly decreased time to ON when compared with levodopa administration (mean 24 vs 61 minutes; P<0.0001) and was associated with fewer dose failures (7% vs 46%) in 88 patients with morning akinesia.1
Newer On-Demand Therapies
Two newer rescue therapies are an appropriate option for on-demand treatment of OFF episodes: inhaled levodopa and sublingual apomorphine. These are discussed in detail in Section 2, Rescue Medications.
Deep Brain Stimulation
Deep brain stimulation surgery may be used for motor fluctuations and dyskinesias in carefully selected patients. Although this option is now used routinely, there is a risk of postoperative complications with this invasive procedure.14,24,30
Nonpharmaceutical Strategies
A low-protein diet may aid the absorption of levodopa and help prevent motor fluctuations.14 Exercise is generally recommended for patients with PD and is documented to help ease motor and nonmotor symptoms, help prevent falls, and improve cognition.3 Physical therapy and occupational therapy may help improve balance, reduce motor problems, and aid the ability to perform activities of daily living.30

Case presentation (cont'd): At a follow-up visit 3 months later, Brian reported no change in his OFF symptoms with continued levodopa, so his neurologist added an MAO-B inhibitor (selegiline) 5 mg twice a day. At a visit 3 months after that, Brian reported that he had a "little bit" of improvement, but his OFF symptoms were still unpredictable and bothersome. His neurologist then switched Brian from carbidopa-levodopa IR to carbidopa-levodopa ER, which helped, but Brian still reported that he had "trouble getting going" in the morning and still had OFF episodes. Both Brian and his wife are now discouraged with the available treatment options and want to try a different approach. Brian's neurologist is concerned that oral medications alone are not able to control Brian's motor fluctuations because of gastroparesis leading to variable medication absorption, which explains Brian's symptoms of delayed ON, dose failures, and morning akinesia. His neurologist reassures Brian that on-demand therapies are available that bypass the gut and quickly take effect to alleviate his symptoms.
Unmet Needs for Rapid-Acting Rescue Medications for OFF Episodes
As PD advances, the therapeutic window for levodopa continues to narrow. Increasing the dosage to improve OFF time does not address the issues with gut absorption. Rescue therapies, defined as treatments with an onset of effect within 15 minutes, are used on demand for OFF episodes, especially those that are disabling, such as morning akinesia or unexpected OFF episodes.3 The ideal rescue medication is a fast-acting, effective, on-demand agent that bypasses the gut and rapidly crosses the blood-brain barrier.3,16 Until recently, apomorphine subcutaneous injection was the only FDA-approved short-acting dopamine agonist for rescue therapy.3
Expert Video Commentary
Section 2: Newer and emerging therapies for motor fluctuations in patients with Parkinson's disease: bench to bedside
Rationale and Unmet Needs
International guidelines indicate that the prevention, delay, and treatment of motor fluctuations and dyskinesias are important priorities in PD care.24 OFF episodes and symptoms vary greatly among patients and may remain unrecognized and untreated until they become disabling.5 Patients with PD have already lost dopaminergic neurons by the time of diagnosis, so the earlier that OFF episodes are treated, the better the likelihood of improved outcomes.5 A survey of 60 patients with PD (experiencing OFF episodes) and 60 caregivers indicated that patients were willing to change therapies in an effort to reduce OFF episodes and improve their quality of life.15
Pathophysiology of PD and Approach to Novel Therapies

Figure 2. Gastroscopy image showing delayed gastric emptying, with levodopa pill still intact in the stomach 1.5 hours after intake.37
Levodopa is the precursor to dopamine.27 In early PD, the response to levodopa is sustained despite its short half-life, presumably because presynaptic nerve terminals are still able to store dopamine. As the disease progresses, there is continued loss of substantia nigra, which reduces the ability to synthesize, store, and release dopamine, leading to fluctuations in therapeutic effect.14 Oral carbidopa-levodopa may have irregular absorption that can lead to fluctuating dopamine synaptic levels, leading to motor complications and oscillation between parkinsonian and dyskinetic states.6
Our understanding of the role of the gastrointestinal tract in PD has evolved markedly during the past decade.37 It is now known that problems with delayed gastric emptying (gastroparesis) are highly prevalent in patients with both early and advanced PD, with prevalence estimated as 70% to 100%.16,37 Delayed ON episodes are likely due to delayed levodopa absorption and slow delivery to the brain as a result of gastroparesis, presence of intestinal protein that competes with the medication for absorption, or pharmacodynamic effects. Dose failures (no-ON) also may occur after meals with protein consumption.16 Imaging studies have revealed intact levodopa tablets in the stomach even 1.5 hours after dosing, which can result in delayed or completely absent therapeutic effect (Figure 2).37
The problem of poor gut absorption explains why merely increasing the oral levodopa dose or adding oral adjunctive therapies may not control delayed ON, dose failures, or suboptimal ON responses. Only medications that bypass the gastrointestinal tract can overcome the problem of poor gut absorption and treat delayed, suboptimal, or absent ON responses in patients with PD.1,16
Recently Approved Therapies for OFF Episodes
Since 2018, the FDA has approved 4 therapies for treating OFF episodes: 2 adjunctive therapies (istradefylline and opicapone) and 2 rescue medications (inhaled levodopa and sublingual apomorphine).38
Adjunctive Therapies
Istradefylline
Istradefylline is an adenosine receptor antagonist indicated as adjunctive treatment to carbidopa-levodopa in adults with PD who are experiencing OFF episodes; it was approved in the United States in 2019.39 It has been approved in Japan since 2013. Istradefylline is considered effective for decreasing OFF time in advanced PD, with efficacy comparable to that of other adjunctive therapies. It has shown good tolerability and particular benefit for patients with nonmotor symptoms, such as depression or cognitive impairment.40 It is administered orally once daily as an adjunctive therapy to carbidopa-levodopa.
Opicapone
Opicapone is a COMT inhibitor indicated as adjunctive treatment to carbidopa-levodopa in patients with PD who are experiencing OFF episodes; it was approved in the United States in 2020.41 It has been approved in Europe since 2016. Opicapone is administered once daily as an adjunct to levodopa.42
Opicapone is a third-generation COMT inhibitor that was developed to improve the effectiveness of COMT inhibitors and reduce liver toxicity.42,43 No adverse events suggesting hepatic toxicity and no relevant changes in liver enzymes have been identified with adjunctive use.42 In a phase 3, randomized 14-week trial, opicapone 50 mg per day significantly reduced OFF time vs placebo, and treatment effect was sustained throughout a 1-year open-label extension.43
Rescue Medications
Two rapid-acting medications have been approved recently for OFF periods: levodopa inhalation powder and apomorphine hydrochloride sublingual (SL) film. Both inhaled levodopa and sublingual apomorphine are self-administered on demand, have an easy route of administration, bypass gut metabolism, and have an onset of action within minutes. These medications are practical for patients who do not like injections. Furthermore, self-administration of any medication is an advantage in the COVID era.11
Levodopa inhalation powder
Levodopa inhalation powder is FDA approved for the intermittent treatment of OFF episodes in patients with PD treated with carbidopa-levodopa.44 This medication was approved in January 2019.45 Inhaled levodopa is an adjunctive medication for patients using carbidopa-levodopa that can be used up to 5 times a day for OFF episodes; it may be especially beneficial for patients with decreased gut motility.29,44 Orally inhaled levodopa uses a patented technology to transform levodopa into an easily inhaled dry powder.45 Inhaled levodopa offers faster absorption and onset of effect compared with oral formulations. Also, unlike conventional dry-powder inhalers, the device used for inhaled levodopa does not require coordination between inhalation and pump activation.46
Inhaled levodopa 84 mg was significantly more effective than placebo in a phase 3 randomized, double-blind trial of OFF episodes with efficacy assessed as mean change in UPDRS motor score 30 minutes after dosing at week 12 (score change –9.83 vs –5.91, respectively; P=0.0088). Efficacy onset was observed as early as 10 minutes for treatment vs placebo (UPDRS score –6.45 vs –4.18; P=0.046), and treatment response was significantly improved through 60 minutes.23 Another randomized, double-blind trial examined inhaled levodopa 84 mg vs placebo for early-morning OFF. Secondary endpoint results showed numerically better treatment efficacy for median time to ON (25.0 vs 35.5 minutes) and ON status at 30 minutes (66.7% vs 44.5%).47 An open-label, randomized, 12-month study confirmed the efficacy of inhaled levodopa for improving motor scores and OFF time vs standard care with oral PD treatment, and documented no clinically significant differences in pulmonary function indices.48
Apomorphine hydrochloride SL film
Apomorphine hydrochloride SL film is a non-ergoline dopamine agonist indicated for the acute intermittent treatment of OFF episodes in patients with PD.49 This medication was approved in May 2020.50 It is self-administered on demand by placing the film under the tongue where it dissolves, without the need for any injection or device assembly.50 The medication is absorbed systemically via the oral mucosa, avoiding first-pass metabolism.22
Apomorphine SL was significantly more effective than placebo in a phase 3, double-blind trial of OFF episodes with efficacy assessed as mean change in MDS-UPDRS motor score 30 minutes after dosing at week 12 (score change –11.1 vs –3.5, respectively; P=0.0002). Full ON response within 30 minutes as rated by patients occurred in 35% vs 16% (P=0.04). Change in MDS-UPDRS score at 15 minutes was also significantly better in treated vs placebo patients (P=0.04), and the significant benefit continued to the last time point measured (90 minutes).22 On-demand treatment with sublingual apomorphine successfully treated motor fluctuations for up to 48 weeks of long-term follow-up, with 84% of patients reporting full ON response by 30 minutes after dosing.51 This rescue therapy is easily administered without the need for an injection device, and multiple studies have documented its rapid onset of effect.52
Emerging Therapies for OFF episodes
Oral Therapies
IPX203 is an investigational oral carbidopa-levodopa ER formulation intended to increase ON time; it is currently in phase 3 trials.29 An open-label, randomized crossover trial found that IPX203 provided more sustained levodopa concentrations, longer duration of effect as measured by MDS-UPDRS scores, greater reduction in OFF time, and greater increase in ON time compared with both carbidopa-levodopa IR and carbidopa-levodopa ER.53
Infusion Therapies
Subcutaneous apomorphine infusion
Subcutaneous apomorphine infusion is an approved therapy in Europe but is not yet approved in the United States. Continuous subcutaneous apomorphine infusion provides constant medication delivery, in comparison with intermittent apomorphine injections.52 A portable pump delivers a continuous dose, most commonly during waking hours, and a rescue bolus can also be administered.52 Apomorphine infusion is a highly effective rescue therapy for reducing motor fluctuations, including OFF episodes, as well as autonomic (nonmotor) manifestations.6,52
Continuous apomorphine subcutaneous infusion was studied in US patients who had PD refractory to other therapies including levodopa intestinal infusion and deep brain stimulation. Infusions reduced daily OFF time and increased ON time without inducing dyskinesias over 12 weeks in a phase 3, open-label study. More than half of the patients (62%) had a reduction of at least 2 hours in daily OFF time after 12 weeks of infusions.54 Efficacy and safety of apomorphine subcutaneous infusion were compared with placebo in TOLEDO, a European randomized, double-blind trial. OFF time was significantly reduced, and the infusion was well tolerated.55
Pump training is necessary for patients and caregivers to prevent adverse events and to enhance adherence to subcutaneous apomorphine infusion.52
Continuous subcutaneous carbidopa-levodopa
Research is ongoing to develop new strategies for continuous subcutaneous delivery of carbidopa-levodopa to reduce OFF time and motor fluctuations.
ABBV-951 is a new soluble formulation of carbidopa and levodopa prodrugs for continuous subcutaneous infusion. It is being evaluated for safety and tolerability (primary endpoints) and for efficacy (exploratory endpoint) in PD patients with motor fluctuations and OFF time not controlled by oral medications.56
ND0612 is a continuous subcutaneous carbidopa-levodopa infusion for treatment of motor fluctuations. A randomized, double-blind phase 3 study is currently comparing ND0612 with active control (oral carbidopa-levodopa IR) to test its efficacy for increasing mean ON time without inducing dyskinesias.57
Strategies for Community Neurologists
Evaluation
Most patients with PD will present first to a community neurologist for management of OFF episodes. Community neurologists should ask patients about levodopa-related motor complications at all visits, including wearing OFF symptoms, ON/OFF symptoms, dyskinesias, and dystonias. Patients should be queried about any falling episodes at least every 6 months and about symptoms of autonomic dysfunction at least once a year.58 HCPs should ask about any psychiatric symptoms, cognitive impairment, and sleep disturbance yearly. Recommendations for rehabilitative therapy and regular exercise should be repeated yearly.58
HCPs should consider whether patients with suboptimal levodopa response have gastrointestinal problems, which may include not only gastroparesis, but also small-intestinal bacterial overgrowth and Helicobacter pylori infection. A team approach to management may be needed, including a dietitian, neurologist, and gastroenterologist.37
Treatment Options
Patients with delayed ON, dose failures, or morning akinesia during levodopa treatment should be educated about the reasons their oral therapies may not be controlling their motor fluctuations, including an explanation about poor absorption due to gastroparesis and protein interference. These patients would be good candidates for on-demand rescue therapies to avoid morning akinesia, delayed ON, dose failures, and unpredictable OFF periods.1 According to a panel of experts, HCPs should encourage patients to try different therapies because no single strategy works for every patient, and no head-to-head studies are available to demonstrate superiority of any agent. Adjunctive therapies and new options should be attempted, such as an alternative oral levodopa medication, non-dopaminergic therapies, or rescue therapies. Many alternative therapies that are already available are underused.59
Shared Decision Making
Treatment strategies must be individualized based on availability of the medication, practicality of use, and patient preferences.46 Shared decision making is documented to improve patient satisfaction and treatment adherence. Using the SHARE approach recommended by the Agency for Healthcare Research and Quality, HCPs should seek patients' participation in health care, work with them to explore treatment options, assess patients' values and preferences, reach a decision together, and evaluate the decision through ongoing follow-up.60 Figure 3 presents a schematic of the shared decision-making pathway.
Whichever therapy is tried, continued follow-up is essential and alternate therapies may be needed.46,59 Complex cases should be referred to a neurologist with expertise in PD treatment.

Figure 3. Patient participation in shared decision making.
Conclusions
Motor and nonmotor fluctuations are highly prevalent complications of levodopa therapy with a variety of manifestations that impair quality of life. Research shows that the earlier OFF episodes are treated, the better patients' outcomes will be. Although the initial strategy is to adjust the levodopa dose or employ adjunctive medications, these options often fall short of preventing an increase in motor fluctuations as PD progresses.
The FDA has recently approved 4 new agents for treating OFF episodes. In addition to current options that they have available, community neurologists may now consider adjunctive therapy with istradefylline or opicapone and rescue therapy with inhaled levodopa or sublingual apomorphine.
Our current understanding of PD highlights the role of the gastrointestinal tract and suggests that only medications that bypass gut metabolism can overcome the problem of poor gut absorption of levodopa and effectively treat delayed, suboptimal, or absent ON responses. Until recently, the only available on-demand medication for OFF episodes was apomorphine subcutaneous injection, but inhaled levodopa and sublingual apomorphine present new options for on-demand rescue therapies that are easily administered and avoid gut metabolism. When making any therapeutic choice, HCPs should educate patients about treatment options and involve them in a shared decision-making process.